Antifungal Selection Tool for Candidemia
Clinical Guidance
Based on IDSA 2023 guidelines for candidemia treatment. Select options below to receive appropriate therapy recommendations.
Hospital wards are wrestling with a silent enemy that’s slipping past traditional defenses: candidemia is a bloodstream infection caused by Candida species, and when the fungus spreads beyond the blood to organs such as the eyes, brain, or kidneys, it becomes a disseminated candida infection. Both conditions carry steep mortality rates, and recent data show they’re climbing faster than many clinicians anticipate. This article breaks down why the threat is growing, who’s most vulnerable, how to catch it early, and what frontline teams can do to keep patients safe.
What Exactly Is Candidemia?
In plain terms, candidemia is the presence of Candida yeast in the bloodstream. While yeast normally lives harmlessly on skin and mucous membranes, it can enter the circulatory system through breaches such as IV catheters, surgical wounds, or a compromised gut barrier. Once in the blood, the organism can hitch a ride to distant organs, turning a relatively simple infection into a full‑blown systemic crisis.
Understanding Disseminated Candida Infections
When Candida moves beyond the bloodstream, it can seed multiple organs-a condition clinicians label disseminated candida infection. Typical sites include the eye (endophthalmitis), brain (meningoencephalitis), heart (endocarditis), and kidneys. The clinical picture is often vague-fever, low blood pressure, or organ‑specific dysfunction-making diagnosis a race against time.
Who’s Most at Risk?
- Intensive Care Unit (ICU) patients: Prolonged ventilation, central venous catheters, and frequent antibiotic use create a perfect storm.
- Those with neutropenia from chemotherapy or bone‑marrow transplants.
- Patients undergoing major abdominal surgery or with severe gastrointestinal perforation.
- Individuals with total parenteral nutrition (TPN) administered through central lines.
- People colonized with resistant species such as Candida auris.
Why Are Cases on the Rise?
Three interlocking trends are driving the surge:
- Increasing use of invasive devices. More patients receive central lines, urinary catheters, and ECMO support, each offering a portal for yeast.
- Broad‑spectrum antibiotic pressure. When bacterial flora are wiped out, Candida faces less competition and can proliferate unchecked.
- Emerging antifungal resistance. Species like Candida auris and fluconazole‑resistant Candida glabrata are spreading worldwide, limiting the effectiveness of first‑line drugs.
Data from the CDC’s 2024 Antimicrobial Resistance Surveillance Program show a 12% annual increase in hospital‑onset candidemia, with mortality climbing from 38% to 45% over the past five years.
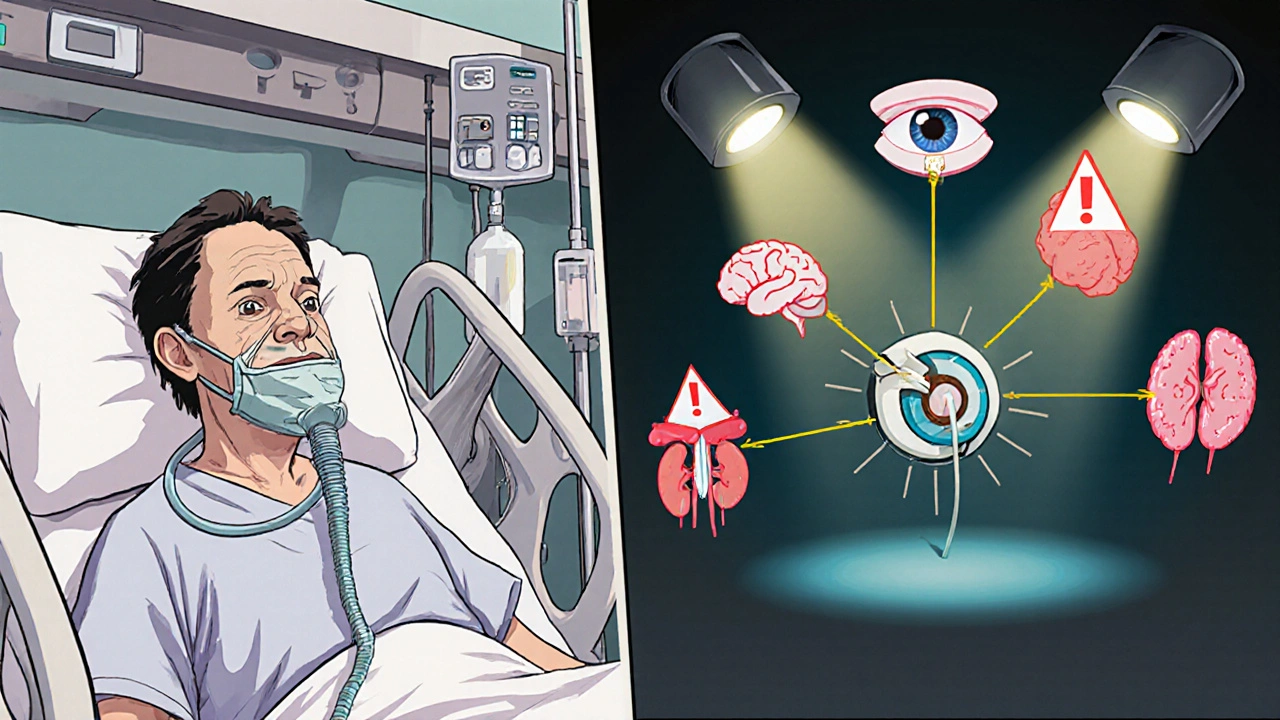
How Do We Diagnose It?
Early detection hinges on a combination of clinical suspicion and rapid laboratory tools.
- Blood cultures. Still the gold standard but can take 48‑72 hours to turn positive.
- Beta‑D‑Glucan assay. Detects fungal cell wall components in serum; a negative result helps rule out invasive candidiasis.
- T2Candida Panel. A PCR‑based test that delivers species‑level identification in 3‑5 hours, dramatically trimming the time to appropriate therapy.
- Imaging. CT or MRI can reveal organ involvement when disseminated infection is suspected, especially for liver, spleen, or brain lesions.
When a patient meets high‑risk criteria (e.g., ICU stay > 7 days, central line in place, broad‑spectrum antibiotics), many institutions now start empiric antifungal therapy while waiting for culture confirmation.
First‑Line Treatment Options
The IDSA 2023 guidelines recommend echinocandins (caspofungin, micafungin, anidulafung) as the preferred initial agents for most adult patients because of their broad spectrum and low resistance rates.
| Class | Representative Drugs | Mechanism | Typical Use | Notable Side Effects |
|---|---|---|---|---|
| Echinocandins | Caspofungin, Micafungin, Anidulafung | Inhibit β‑1,3‑glucan synthesis | First‑line for most adults | Elevated liver enzymes, infusion reactions |
| Azoles | Fluconazole, Voriconazole, Itraconazole | Inhibit ergosterol synthesis (lanosterol 14α‑demethylase) | Step‑down after stabilization; specific species (C. albicans) susceptible | Hepatotoxicity, QT prolongation, drug‑drug interactions |
| Polyenes | Amphotericin B (deoxycholate, lipid formulations) | Bind ergosterol, create membrane pores | Rescue therapy for resistant strains | Nephrotoxicity, infusion fever |
If a Candida species shows resistance to echinocandins (rare but reported in C. auris), high‑dose fluconazole or lipid‑formulation amphotericin B become alternatives. Therapeutic drug monitoring is essential for azoles to avoid toxicity.
Preventing Hospital‑Acquired Candidemia
Prevention is a team sport. Hospitals that have cut candidemia rates by half rely on a bundle of interventions:
- Catheter hygiene protocols. Daily review of line necessity, antimicrobial‑impregnated catheters, and chlorhexidine skin prep.
- Antifungal stewardship. Restrict empiric azole use, enforce de‑escalation based on culture data.
- Environmental cleaning. UV‑C disinfection and dedicated equipment for rooms housing C. auris patients.
- Rapid diagnostics. Implementing T2Candida or MALDI‑TOF on positive cultures shortens time to targeted therapy.
- Nutrition management. Minimize TPN duration and use peripheral lines when possible.
Education of bedside nurses and physicians about early signs-persistent fever despite antibiotics, new organ dysfunction-also raises the index of suspicion.
Emerging Threat: Candida auris
First identified in 2009, C. auris is now a global concern. It thrives on hospital surfaces, resists many disinfectants, and shows multi‑drug resistance (often to fluconazole, amphotericin B, and sometimes echinocandins). Outbreaks in UK intensive care units have prompted the NHS to issue specific infection‑control guidance, including mandatory screening of contacts and cohort isolation.
Rapid identification using MALDI‑TOF or whole‑genome sequencing is critical; misidentification as C. parapsilosis can lead to ineffective therapy.
Key Takeaways Checklist
- High‑risk patients: ICU, central lines, neutropenia, TPN.
- Watch for persistent fever > 48 h despite broad‑spectrum antibiotics.
- Order blood cultures plus beta‑D‑glucan or T2Candida for faster results.
- Start echinocandin empirically; switch to azole only after susceptibility confirmed.
- Implement catheter‑care bundles and antifungal stewardship to curb incidence.
- Be vigilant for C. auris; isolate promptly and clean environment thoroughly.
Frequently Asked Questions
What is the difference between candidemia and a Candida bloodstream infection?
Both terms describe Candida in the blood. “Candidemia” is the clinical diagnosis; “Candida bloodstream infection” is a broader phrase that includes the same condition. In practice they are used interchangeably.
How long does it take for blood cultures to grow Candida?
Standard aerobic bottles usually become positive within 48‑72 hours, but some slow‑growing species may need up to 5 days.
Can candidemia be prevented without removing central lines?
Complete avoidance is rare, but stringent insertion techniques, chlorhexidine dressings, and daily necessity checks can dramatically lower risk while keeping the line in place when it’s essential.
When should I switch from an echinocandin to fluconazole?
After blood cultures identify a fluconazole‑susceptible species (e.g., C. albicans) and the patient is clinically stable, a step‑down to oral fluconazole is recommended, typically after 5‑7 days of IV therapy.
Is Candida auris more deadly than other Candida species?
Mortality rates for C. auris range from 30‑60 % depending on the outbreak and treatment delays, which is comparable or slightly higher than traditional species due to resistance and delayed appropriate therapy.
What role does antifungal stewardship play in controlling candidemia?
Stewardship programs audit antifungal prescriptions, ensure drugs are used only when indicated, and promote early de‑escalation. Hospitals with active stewardship have reported up to a 40 % reduction in candidemia incidence.
9 Comments
Write a comment
More Articles

Fiber Supplements and Medications: When to Take Them to Avoid Absorption Problems
Fiber supplements can block the absorption of key medications like thyroid pills, blood thinners, and antibiotics. Learn the safe timing rules to avoid dangerous interactions and keep your meds working properly.

Top 5 Thyroid Medication Alternatives to Synthroid in 2024
Explore five alternatives to Synthroid, a commonly prescribed thyroid medication. This guide delves into the characteristics, benefits, and potential downsides of each option. From natural desiccated thyroid extracts like Armour Thyroid and Nature-Throid to synthetic options such as Cytomel and Tirosint, we cover the essential information you need. Use this resource to understand which alternative might be more suitable for your thyroid health needs. Make a well-informed decision about your health with this comprehensive analysis.

Sarah Unrath
October 19, 2025 AT 20:53Candidemia is real and we need better line care