
Hepatitis B Treatment Comparison Tool
Epivir HBV (Lamivudine)
First-line treatment since 1998, affordable and easy to take. However, resistance develops in ~30% of patients within 3 years.
High Resistance RiskTenofovir (TDF/TAF)
Highest barrier to resistance, deep viral suppression, and excellent safety profile. TAF has fewer kidney and bone impacts.
Low Resistance RiskEntecavir
High potency with low resistance risk. Ideal for treatment-naive patients but requires careful monitoring in certain cases.
Low Resistance RiskAdefovir
Less commonly used due to moderate efficacy and potential for kidney toxicity. Reserved for special cases.
Moderate Resistance RiskTreatment Comparison Table
| Drug | Undetectable HBV DNA (48 weeks) | Resistance Rate (5 years) | Key Side Effects | Typical Dose |
|---|---|---|---|---|
| Epivir HBV (Lamivudine) | 70-80% | ≈30% | Nausea, headache; low renal impact | 100 mg once daily |
| Tenofovir Disoproxil Fumarate (TDF) | ≈95% | < 5% | Can lower eGFR; monitor kidney function | 300 mg once daily |
| Tenofovir Alafenamide (TAF) | ≈95% | < 5% | Minimal kidney impact; slight lipid elevation | 25 mg once daily |
| Entecavir | ≈90% | < 5% | Mild dizziness; rare lactic acidosis | 0.5 mg once daily |
| Adefovir | ≈70% | ≈20% | Higher risk of nephrotoxicity | 10 mg once daily |
| Pegylated Interferon-α | ≈40-50% | Not applicable | Flu-like symptoms, depression, thyroid dysfunction | 180 mcg once weekly |
When you or a loved one is diagnosed with chronic hepatitis B, the first question is usually: “Which pill works best?” Epivir HBV (lamivudine) has been on the market fordecades, but newer antivirals promise stronger viral suppression and lower resistance risk. This guide breaks down lamivudine side‑by‑side with the most prescribed alternatives, so you can see which option matches your health goals, virus genotype, and lifestyle.
Key Takeaways
- Lamivudine is cheap and once‑daily, but resistance can appear after 2‑3years of therapy.
- Tenofovir (both TDF and TAF) offers the highest barrier to resistance and the deepest viral suppression.
- Entecavir is a solid middle ground-high potency with a low resistance profile for treatment‑naïve patients.
- Adefovir is rarely first‑line now because of modest efficacy and kidney‑related side effects.
- Pegylated interferon‑α provides a finite treatment course and may lead to seroconversion, but it carries flu‑like side effects and injection discomfort.
What Is Epivir HBV (Lamivudine)?
Lamivudine is a nucleoside analogue that targets the hepatitis B virus (HBV) DNA polymerase, interrupting viral replication. Commercially sold as EpivirHBV, the drug received FDA approval in 1998 and quickly became the go‑to oral therapy because of its simple once‑daily dosing and low cost.
Lamivudine’s chemistry is similar to the HIV drug of the same name, allowing it to block the reverse‑transcriptase activity that HBV relies on. In clinical trials, 70‑80% of treatment‑naïve patients achieved undetectable HBV DNA after 48weeks, but long‑term use raised concerns about viral resistance.
How Lamivudine Works (and Where It Falls Short)
Lamivudine competes with the natural nucleoside cytidine during viral DNA synthesis. Once incorporated, it terminates the growing DNA chain, halting replication. The simplicity of this mechanism makes dosing easy-usually 100mg oral tablet once daily.
Unfortunately, HBV’s error‑prone polymerase can quickly mutate the YMDD motif, rendering lamivudine ineffective. Studies published in2022 show that up to 30% of patients develop the YMDD resistance mutation after three years of continuous therapy. When resistance emerges, viral load rebounds and liver inflammation may flare.
Top Alternatives to Lamivudine
Over the past decade, three drugs have become the de‑facto first‑line choices for chronic HBV: tenofovir (both TDF and the newer TAF), entecavir, and pegylated interferon‑α. Adefovir still appears in older guidelines but is now a niche option.
Tenofovir disoproxil fumarate (TDF) is a nucleotide analogue that blocks HBV polymerase with a higher genetic barrier to resistance than lamivudine. Recommended dose is 300mg daily.
Tenofovir alafenamide (TAF) delivers the same active molecule as TDF but at a lower dose (25mg) and with reduced kidney and bone toxicity.
Entecavir is a guanosine analogue that inhibits three steps of the HBV replication cycle. Dosing is 0.5mg daily for treatment‑naïve patients.
Adefovir dipivoxil is an older nucleotide analogue (10mg daily) that needs dose adjustment in renal impairment.
Pegylated interferon‑α is an injectable cytokine given once weekly for 48weeks; it stimulates the immune system to clear infected cells.
Side‑Effect Profiles at a Glance
All HBV antivirals share some common concerns-fatigue, headache, and occasional gastrointestinal upset-but the severity and organ‑specific risks differ.
- Lamivudine: Generally well tolerated; rare nausea, headache.
- TDF: Can lower eGFR and cause phosphate wasting; monitor kidney function.
- TAF: Minimal kidney impact; slight lipid elevation reported.
- Entecavir: Mild dizziness; rare lactic acidosis in patients with mitochondrial disorders.
- Adefovir: Higher risk of nephrotoxicity, especially at >10mg.
- Pegylated interferon‑α: Flu‑like symptoms, depression, thyroid dysfunction; requires close psychiatric monitoring.
Head‑to‑Head Comparison Table
| Drug | Undetectable HBV DNA (48wks) | Resistance rate (5yrs) | Key side effects | Typical dose |
|---|---|---|---|---|
| Lamivudine (EpivirHBV) | 70‑80% | ≈30% | Nausea, headache; low renal impact | 100mgoncedaily |
| Tenofovirdisoproxilfumarate (TDF) | ≈95% | <1% | Renal ↓eGFR, bone mineral loss | 300mgoncedaily |
| Tenofoviralafenamide (TAF) | ≈95% | <1% | Minor lipid rise, mild liver enzyme increase | 25mgoncedaily |
| Entecavir | ≈90‑95% | ≈1‑2% (treatment‑naïve) | Dizziness, rare lactic acidosis | 0.5mgoncedaily |
| Adefovir dipivoxil | ≈55‑65% | ≈10‑15% | Nephrotoxicity, phosphate loss | 10mgoncedaily |
| Pegylated interferon‑α | ≈20‑30% HBsAg loss | Not applicable (finite course) | Flu‑like, depression, thyroid issues | 180µgSConceweekly (48wks) |
Choosing the Right Therapy for You
Deciding between lamivudine and its alternatives isn’t a one‑size‑fits‑all answer. Consider these practical factors:
- Baseline viral load. Patients with >1,000,000IU/mL benefit most from tenofovir or entecavir, which achieve deeper suppression.
- Kidney function. If eGFR<60mL/min, avoid TDF and consider TAF or entecavir.
- Cost and insurance coverage. Lamivudine remains the cheapest generic; newer agents may require prior‑authorisation.
- Future pregnancy plans. Tenofovir (both forms) is safe in pregnancy, while interferon is contraindicated.
- Resistance history. Prior lamivudine exposure raises the bar for resistance-switching to tenofovir or entecavir is advisable.
Ultimately, a hepatologist will weigh these variables alongside liver fibrosis stage (often measured by FibroScan) to tailor therapy.
Managing Resistance and Switching Strategies
If you’ve been on lamivudine for several years and your HBV DNA starts climbing, the first step is a resistance test for the YMDD mutation. When resistance is confirmed, guidelines recommend an immediate switch to a high‑barrier drug-typically tenofovir (TDF or TAF) or entecavir.
Switching is straightforward: continue lamivudine until the new drug reaches therapeutic levels (usually one week), then discontinue lamivudine. Monitoring liver enzymes and viral load every 12weeks helps confirm the transition’s success.
For patients who cannot tolerate tenofovir because of renal issues, entecavir is the preferred rescue option. In rare cases where both tenofovir and entecavir have failed, combination therapy (tenofovir+entecavir) or enrollment in clinical trials for novel capsid inhibitors may be considered.
Future Directions in HBV Therapy
Research in 2025 is shifting from indefinite suppression toward functional cure-meaning loss of hepatitis B surface antigen (HBsAg) without relapse. New agents under investigation include:
- RNA interference (RNAi) therapies that silence HBV transcripts.
- Capsid assembly modulators that prevent viral particle formation.
- Therapeutic vaccines aiming to boost the immune response.
While these options aren’t widely available yet, they could eventually replace current nucleos(t)ide analogues, making the choice between lamivudine and newer drugs a historical footnote.
Frequently Asked Questions
Can I take lamivudine and tenofovir together?
Combination therapy is rarely needed for treatment‑naïve patients because tenofovir alone provides strong viral suppression. The combo may be used in rescue situations when resistance to both agents is documented, but this should only be done under specialist supervision.
Is lamivudine safe for long‑term use?
Lamivudine is well tolerated, but the risk of YMDD‑mediated resistance rises sharply after two years. Long‑term use is generally discouraged in patients with high viral loads or prior resistance.
Which drug is best for a patient with chronic kidney disease?
Tenofovir alafenamide (TAF) is preferred because it delivers the active drug at lower systemic concentrations, sparing the kidneys. If TAF is unavailable, entecavir is an acceptable alternative.
Does pegylated interferon‑α cure hepatitis B?
Interferon can lead to HBsAg loss in about 20‑30% of patients, which is considered a functional cure. However, the therapy is intensive, has notable side effects, and is not suitable for everyone.
How often should I get labs while on HBV therapy?
For nucleos(t)ide analogues like lamivudine, tenofovir, or entecavir, check ALT, HBV DNA, and renal function every 12weeks for the first year, then every 6-12months. Interferon patients need more frequent monitoring during the 48‑week course.
Choosing between lamivudine and newer HBV antivirals boils down to three core questions: How strong does viral suppression need to be? What’s the patient’s kidney and bone health status? And how does cost or insurance coverage influence the decision? By answering these, you can move from guesswork to a therapy plan that keeps the virus in check and protects the liver for the long haul.
15 Comments
Write a comment
More Articles
Tips for preventing fungal infections and the need for ketoconazole treatment
In my latest blog, I've shared some handy tips for preventing fungal infections so you can potentially avoid the need for treatments like ketoconazole. We dive into the importance of maintaining good hygiene, keeping your skin dry and clean, and wearing breathable fabrics to prevent a conducive environment for fungi. We also touch on the importance of a healthy diet to boost your immune system. If you do get an infection, don't panic because treatments like ketoconazole are there to help. But remember, prevention is always better than cure!

Tadacip (Tadalafil) vs Other ED Pills: Complete Comparison Guide
A thorough comparison of Tadacip (tadalafil) with sildenafil, vardenafil, avanafil, and generic tadalafil, covering onset, duration, side effects, cost and how to choose the best ED pill.
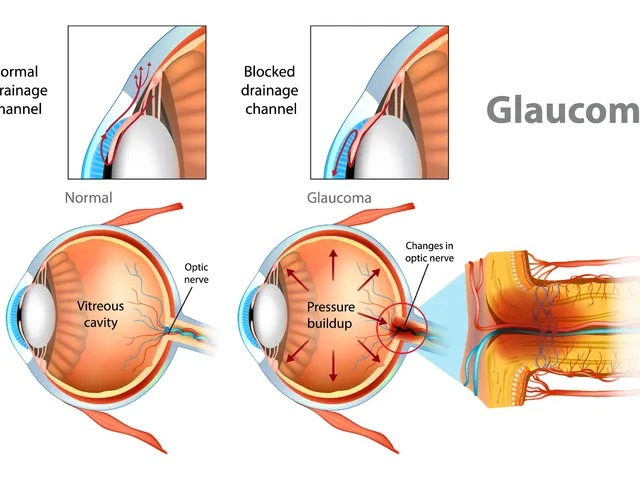
The effectiveness of dorzolamide in treating different types of glaucoma
Hey there, gentlemen! I just wanted to share some insights on a very interesting topic - the effectiveness of dorzolamide in treating different types of glaucoma. We'll dive into its mechanism of action, its beneficial effects on different types of this eye condition, and why it's becoming a go-to medication. Through this, I hope to provide everyone with a deeper understanding of this treatment option in a clear and simple language. Remember, knowledge is your best ally in health matters!
Jonathan Alvarenga
October 5, 2025 AT 13:34Alright, let me just wade through this sea of hype and tell you why Epivir HBV (Lamivudine) feels like the cheap knock‑off you get at a discount store while the rest of the newer antivirals are the luxury models you actually want to drive. First off, the cost is undeniably attractive – you can snag a bottle for pennies compared to the hefty price tags on Tenofovir or Entecavir, and that’s why it still hangs around in budget‑conscious clinics. But cheap comes with a catch: the resistance rate is hovering around thirty percent after just a few years, which is basically a ticking time‑bomb for any patient hoping for long‑term suppression. You take lamivudine, feel all warm and fuzzy for a while, then bam – the virus mutates, the YMDD motif flips, and you’re back to square one, often needing to jump to a more aggressive regimen anyway. The efficacy numbers in the guide – 70 to 80 percent undetectable HBV DNA at 48 weeks – are respectable but pale next to the ninety‑plus percent you see with TDF or TAF. Sure, the side‑effect profile looks benign, but when you factor in the inevitable need for rescue therapy, the cumulative toxicity can actually surpass that of the “cleaner” drugs. Moreover, the adherence story isn’t as simple as once‑daily dosing; patients who develop resistance often experience flares, which can erode trust in any medication. In contrast, Tenofovir’s barrier to resistance is under five percent, making it the gold standard for durability, especially in patients with high viral loads. Entecavir also boasts a low resistance rate and high potency, positioning it as the middle child that gets the best of both worlds. The guide’s table nicely outlines the differences, but it underplays the real‑world impact of renal monitoring required for TDF – a point that some clinicians brush aside as “just a lab.” And don’t even get me started on the lipid changes with TAF; while they’re mild, they’re still a reminder that nothing is truly side‑effect free. If your primary goal is to avoid resistance and you can afford the price, why cling to a drug whose biggest selling point is its cheapness? In the end, lamivudine can be a stop‑gap for patients who absolutely cannot access pricier options, but for anyone with the means, it’s a gamble that most modern guidelines advise against.