
When you walk into a pharmacy and find your prescription out of stock, it’s not a glitch. It’s a symptom of a global system that’s been stretched too thin. The truth is, international supply chains are the invisible backbone of modern medicine-and they’re breaking under pressure. Over 80% of the active ingredients in U.S. prescription drugs are made overseas, mostly in China and India. And when geopolitical tensions, port closures, or shipping delays hit, patients pay the price. This isn’t about politics. It’s about physics: if the chain snaps, the medicine stops moving.
How Did We Get Here?
In the 1990s, pharmaceutical companies didn’t just outsource manufacturing-they outsourced entire production systems. Why? Because it was cheaper. Labor in India and China cost a fraction of what it did in the U.S. or Europe. Regulatory oversight was looser. Taxes were lower. By 2025, over 50% of global pharmaceutical manufacturing output came from just two countries. That sounds efficient. Until it isn’t.Take the case of heparin, a blood thinner used in every hospital. In 2008, a contaminated batch from China caused over 80 deaths in the U.S. That should’ve been a wake-up call. Instead, companies doubled down on cost-cutting. By 2025, 94% of multinational drugmakers still relied on single-source suppliers for critical ingredients. That’s like building a bridge with only one steel cable. One snap, and everything falls.
The Domino Effect of Disruptions
In 2024, a port strike in Shanghai shut down 17 major pharmaceutical shipping lanes for 45 days. No one noticed until hospitals in Ohio and Georgia ran out of antibiotics. The delay wasn’t because the drugs weren’t made-it was because the containers sat on docks while customs paperwork got stuck in bureaucratic limbo. That’s the hidden cost of globalization: complexity.Lead times from China to the U.S. have increased by 50% since 2019. That means if a factory in Guangdong shuts down for a week, it takes over a month for the ripple to reach a pharmacy shelf. Meanwhile, U.S. logistics costs hit $2.3 trillion in 2025-8.7% of the entire national GDP. That’s not just shipping fees. It’s the cost of keeping extra inventory, paying for air freight, and scrambling to find backup suppliers.
And it’s getting worse. The U.S. imposed 12 new tariff categories on Chinese pharmaceutical inputs between 2024 and 2025. Those tariffs didn’t stop imports-they just made them more expensive. One generic diabetes drug saw a 280% price jump overnight. Patients skipped doses. Hospitals rationed. That’s not speculation. That’s data from the American Pharmacists Association’s 2025 report.
Who’s Getting Hit the Hardest?
It’s not just big-name drugs. The shortages are most dangerous for older, generic medications-things like insulin, epinephrine, and antibiotics. These aren’t high-margin products. They’re low-cost, high-volume essentials. And because they’re cheap, companies stopped investing in local production. Why build a factory when you can buy from a supplier who’s 70% cheaper?But here’s the catch: 90% of global businesses are small or mid-sized manufacturers. They don’t have the cash to stockpile six months of inventory. When a supplier in India faces a power outage or a Chinese factory gets hit by flooding, these smaller drugmakers can’t pivot. They just go dark. That’s why 56% of companies surveyed by the National Foreign Trade Council in 2025 had to delay or cancel product launches. That’s not a business problem. It’s a public health crisis.
The New Rules: Multi-Shoring and Nearshoring
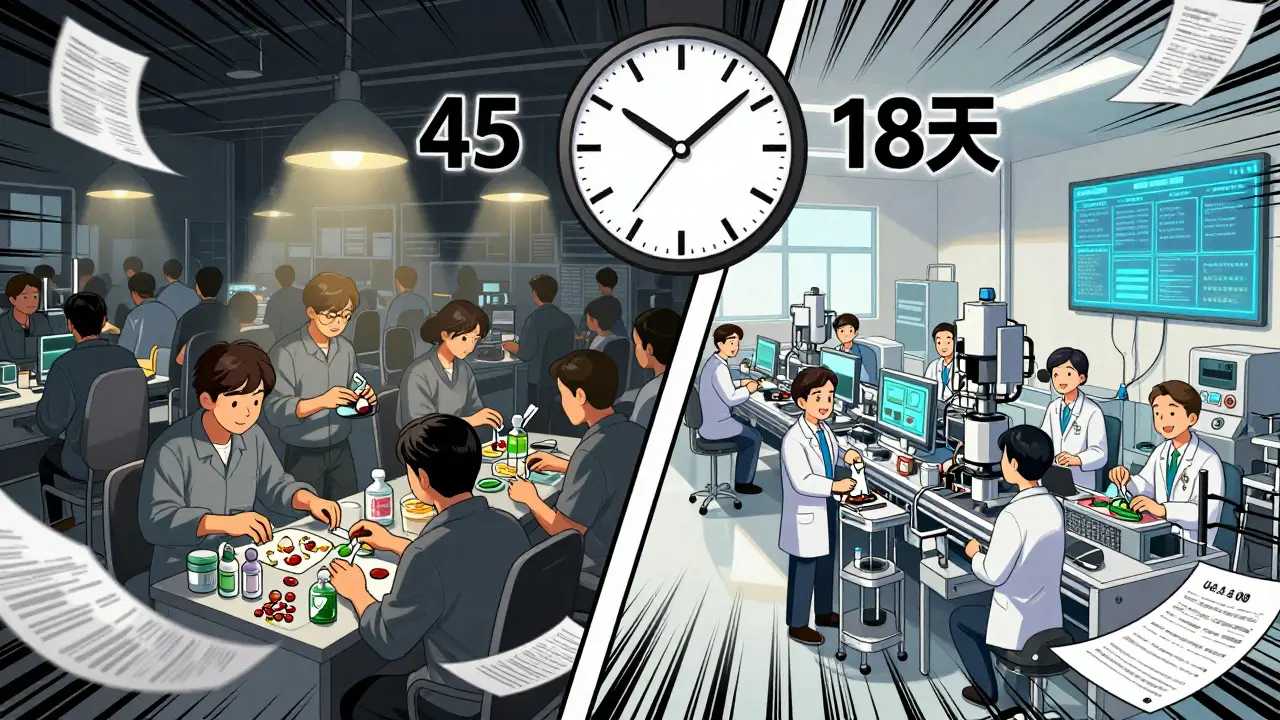
The smartest companies aren’t trying to bring everything back to the U.S. They’re spreading out. This is called multi-shoring: making the same drug in three different places-say, one in India, one in Mexico, and one in Poland. It’s not cheap. Setting up a second manufacturing line costs 22% of your annual procurement budget. But when disruption hits, your backup kicks in.One Fortune 500 medical device maker cut its drug shortage risk by 70% by moving 40% of its production to Mexico. Transportation costs dropped 35%. Lead times shrank from 45 days to 18. And because Mexico is part of the USMCA trade deal, tariffs stayed stable. That’s the sweet spot: geographic diversity without sacrificing speed.
And it’s working. By 2025, 78% of pharmaceutical firms had adopted supplier diversification-up from 35% in 2020. Companies that did this saw 65% fewer disruption days per year. That’s not a minor improvement. That’s the difference between a hospital having enough insulin and a patient going without.
Technology Isn’t a Magic Fix
You’ve heard about AI, blockchain, and digital twins. They’re real. And they’re helping. A company in Birmingham, Alabama, used AI-powered demand forecasting to reduce its inventory waste by 30%. Another in Tennessee used blockchain to verify the origin of raw materials-cutting quality disputes by 65%. But here’s the truth: technology doesn’t fix bad strategy.Too many companies think buying a fancy software system will solve their supply chain problems. It won’t. If your only supplier is in a region prone to floods or political unrest, no algorithm can prevent a shortage. Digital tools help you see the problem faster. They don’t stop it.
And the workforce gap is real. 33% of global trade managers are understaffed in 2025. You can have the best AI in the world, but if no one knows how to use it-or if your customs broker is overworked and missing filings-you’re still vulnerable.

The Cost of Doing Nothing
Some experts argue reshoring is too expensive. Professor Richard Baldwin of IMD Business School points out that U.S. manufacturing wages are 4.8 times higher than in China. He’s right. But he’s missing the bigger picture. The cost of a shortage isn’t just dollars. It’s lives.In 2024, a single shortage of a critical antibiotic led to 120 extra deaths in U.S. hospitals, according to CDC data. That’s not an abstract number. That’s 120 families. That’s 120 funerals. When you’re choosing between paying $2 more per pill and risking a patient’s life, the math changes.
And the economic toll is mounting. The OECD cut its 2025 global GDP forecast to 2.9%-partly because trade barriers are slowing the flow of medical goods. That’s not just about cars or phones. It’s about the medicines that keep people alive.
What’s Next?
The path forward isn’t about going back. It’s about going smart. Governments need to stop treating supply chains like trade policy problems. They’re public health systems. The U.S. should incentivize domestic production of critical drugs-not through subsidies, but through guaranteed purchase agreements. If a company builds a factory to make life-saving antibiotics, the government buys a fixed amount every year. That gives companies certainty. That’s how you build resilience.For manufacturers, the answer is diversification. Don’t rely on one country. Don’t rely on one supplier. Build redundancy into your network. Use nearshoring where it makes sense. Invest in digital tools-not to replace people, but to empower them.
And for patients? Demand transparency. Ask your pharmacist: Where is this drug made? If they don’t know, it’s time to ask harder questions.
Why are so many drugs made in China and India?
China and India dominate drug manufacturing because they offer low labor costs, large-scale production capacity, and government support for chemical and pharmaceutical industries. For decades, U.S. and European drugmakers outsourced production to cut costs. Today, over 80% of active pharmaceutical ingredients (APIs) come from these two countries. While this kept drug prices low, it also created extreme dependency-making the entire system vulnerable to any single disruption, whether it’s a port strike, a natural disaster, or a trade war.
Can the U.S. make its own drugs again?
Yes, but not overnight. Building a domestic pharmaceutical manufacturing base requires billions in investment, trained workers, and regulatory approvals that take years. The U.S. has started with targeted efforts-like the Biomanufacturing and Innovation Initiative-which funds domestic production of critical drugs like insulin and epinephrine. But scaling this to cover all essential medicines would take a decade and major policy shifts. The goal isn’t to eliminate foreign suppliers-it’s to build backup capacity so no single country holds the entire supply chain hostage.
How do tariffs affect drug shortages?
Tariffs don’t stop imports-they just make them more expensive. When the U.S. imposed new tariffs on Chinese pharmaceutical inputs in 2024-2025, many manufacturers didn’t stop buying. Instead, they passed the cost to hospitals and patients. One study found that tariff-affected drugs saw price increases of 200-300%. That led to reduced usage, rationing, and even stockouts. In effect, tariffs made drugs harder to afford and harder to get, worsening shortages instead of fixing them.
What’s the difference between nearshoring and reshoring?
Nearshoring means moving production to a nearby country-like shifting from China to Mexico or from India to Poland. It keeps costs lower than reshoring (bringing production back to the U.S.) while cutting shipping times and reducing geopolitical risk. Reshoring means bringing manufacturing home, which is more expensive but offers the highest level of control. Most companies are choosing nearshoring because it’s a practical middle ground: faster, cheaper, and more resilient than relying on Asia alone.
Are drug shortages getting worse?
Yes, and they’re becoming more frequent and longer-lasting. In 2024, the FDA recorded over 250 active drug shortages, up from 120 in 2020. The average duration of a shortage rose from 11 months in 2020 to 18 months in 2025. Climate events, geopolitical conflicts, and workforce gaps are all contributing. What used to be occasional hiccups are now systemic failures. The trend won’t reverse without structural changes to how drugs are produced and distributed globally.
13 Comments
Write a comment
More Articles

Decadron Explained: Uses, Dosage, Side Effects & Safety Tips
A clear guide to Decadron - what it is, how it works, proper dosing, common side effects and safety advice for everyday use.

Rise of Next-Gen Viagra Alternatives in 2025: What You Need to Know
Curious about what's next after Viagra? This article digs into the 2025 wave of new alternatives, including advanced PDE5 inhibitors and innovative therapies shaking up erectile dysfunction treatment. Explore real results, learn what works, and get savvy about what's coming to pharmacy shelves soon. Whether you're after faster effects or fewer side effects, this guide helps you cut through the marketing and find the best science-backed options. Don't get stuck in the past; see how sexual health is evolving this year.
Vamsi Krishna
February 13, 2026 AT 00:51Look, I get it - you're scared of China. But let's be real: 80% of APIs come from India and China because they've built the most efficient, scalable, and technically advanced manufacturing ecosystems on the planet. You think building a factory in Ohio with union wages and EPA compliance is gonna make penicillin cheaper? Nah. It'll make it unaffordable. We didn't 'outsource' because we were lazy - we outsourced because it made sense. Now you want to pay $300 for insulin because you're mad at Xi? That's not resilience. That's nationalism with a side of delusion.