When a patient shows an unexpected reaction after starting a new medication, clinicians need a reliable way to decide if the drug is really to blame. That decision‑making process is called Naranjo Scale is a standardized questionnaire that estimates the probability that a drug caused an adverse event. By turning a clinical judgment into a simple score, the scale helps doctors, pharmacists, and regulators document adverse drug reactions (Adverse Drug Reaction is a harmful or unintended response to a medication at normal doses) in a reproducible way.
Why a Formal Causality Tool Matters
Before the 1960s thalidomide tragedy, most drug‑safety reports relied on anecdotal impressions. Modern pharmacovigilance demands objective evidence because regulatory agencies-such as the Food and Drug Administration (FDA) is a U.S. agency that oversees drug safety and efficacy and the European Medicines Agency (EMA) is a EU body that coordinates drug safety monitoring across member states-require structured assessments for serious ADRs. A structured tool reduces bias, improves reporting consistency, and makes data usable for signal detection across borders.
History of the Naranjo Scale
Developed by Carlos A. Naranjo and colleagues, the scale first appeared in Clinical Pharmacology & Therapeutics in August 1981. It was a direct response to the post‑thalidomide need for a quick, paper‑based method that could be used in any clinical setting, from busy emergency rooms to academic research labs. Over four decades, the scale has become the most cited causality tool, featuring in 78 % of ADR case reports published in 2022.
How the Scale Works: The Ten Questions
Each question receives a score of +2, +1, 0, or ‑1 based on the clinician’s answer (Yes, No, or ‘Do Not Know’). The total determines the likelihood category.
- Are there previous conclusive reports on this reaction? (+1/0/0)
- Did the adverse event appear after the suspected drug was given? (+2/‑1/0)
- Did the reaction improve when the drug was discontinued? (+1/0/0)
- Did the reaction reappear when the drug was re‑administered? (+2/‑1/0)
- Are there alternative causes that could have caused the reaction? (‑1/+2/0)
- Was a placebo given to see if the reaction recurred? (‑1/+1/0)
- Was the drug level in the toxic range? (+1/0/0)
- Was there a dose‑response relationship? (+1/0/0)
- Did the patient have a similar reaction to the same or similar drug in the past? (+1/0/0)
- Is there objective evidence (lab test, imaging) confirming the reaction? (+1/0/0)
The score interpretation is:
- ≥ 9 = Definite ADR
- 5‑8 = Probable ADR
- 1‑4 = Possible ADR
- ≤ 0 = Doubtful ADR
Step‑by‑Step Application in Clinical Practice
Follow this quick workflow to integrate the Naranjo Scale into daily practice:
- Collect the case details. Gather medication timeline, dosage, lab values, and any prior similar events.
- Answer the ten questions. Use a printed worksheet or a digital calculator (many EHRs, such as Epic Systems is a widely used EHR platform that can auto‑populate several Naranjo questions can fill in items like drug start date and lab results automatically.
- Calculate the total score. Add up the points. If you’re using a spreadsheet, a simple SUM formula does the job; for Python fans, the open‑source Naranjo Calculator on GitHub processes 100 assessments per minute with 100 % accuracy.
- Interpret the result. Place the case into one of the four categories and document the rationale for each answer, especially any “Do Not Know” items.
- Report the ADR. Submit the structured assessment to the national pharmacovigilance centre (e.g., FDA’s FAERS or WHO’s VigiBase) as part of the mandatory safety report.
Training typically requires 2‑4 hours of classroom instruction plus hands‑on practice with 20‑30 real cases. After that, most clinicians achieve reliable scores within 5 minutes per case.

How It Stacks Up Against Other Tools
| Feature | Naranjo Scale | WHO‑UMC System is a categorical causality method from the World Health Organization‑Uppsala Monitoring Centre | Liverpool ADR Probability Scale is a tool designed for multiple‑drug assessments in polypharmacy |
|---|---|---|---|
| Scoring method | Numeric (‑1 to +2 per question) | Categorical (certain, probable, possible, unlikely) | Numeric with multi‑drug weighting |
| Number of questions | 10 | 6 (qualitative statements) | 12 with drug‑specific modifiers |
| Inter‑rater reliability (kappa) | 0.4‑0.6 (moderate) | 0.2‑0.4 (low) | 0.5‑0.7 (higher for polypharmacy) |
| Best for | Single‑drug reactions, quick bedside use | Broad regulatory reporting, low‑resource settings | Elderly patients on many meds, research studies |
| Major limitation | Cannot handle multiple concurrent drugs | Lacks numerical precision | More complex, needs training |
In practice, many institutions start with the Naranjo Scale for its simplicity, then switch to the Liverpool Scale when dealing with frail, poly‑medicated patients.
Strengths, Weaknesses, and Common Pitfalls
What clinicians love: The score forces you to think through every piece of evidence, making the assessment less likely to be swayed by intuition alone. It also fits on a single A4 sheet, which is handy for paper charts.
Where it trips up: Questions about rechallenge (Q4) and placebo administration (Q6) are often impossible to answer ethically, so they default to “Do Not Know” and drag the total down. Also, the binary “Yes/No” format can miss nuanced clinical reasoning-two equally plausible explanations may both be marked “No,” skewing the score toward “possible.”
To avoid these pitfalls, document the rationale for every “Do Not Know” and, when feasible, supplement the Naranjo result with a second tool (e.g., ALDEN for Stevens‑Johnson syndrome) or a narrative assessment.
Digital Tools and Automation
Beyond the classic paper worksheet, several digital options speed up the workflow:
- Python Naranjo Calculator. A console app released in 2023 processes up to 100 cases per minute and validates each response.
- EHR integration. Epic’s Safety Module auto‑populates four of the ten questions from medication orders and lab data, cutting average completion time from 11 minutes to 4 minutes.
- Mobile apps. Free Android and iOS apps let clinicians score on the go, with built‑in prompts to avoid common scoring errors.
Automation lowers transcription mistakes-one 2023 Cureus study reported error rates dropping from 28 % on paper to 9 % with the digital calculator.
Regulatory Acceptance and Global Adoption
Regulators consider the Naranjo Scale an acceptable method for initial causality evaluation. The FDA’s 21 CFR 310.305 explicitly references structured tools, and the EMA’s Good Pharmacovigilance Practices (GVP) Module VI cites the scale as a recommended approach. According to the 2022 WHO Pharmacovigilance Report, 78 % of participating countries use the Naranjo Scale in their national reporting systems, with adoption rates over 90 % in North America and Europe.
Its popularity isn’t limited to high‑income regions. In Asia‑Pacific, 76 % of hospitals reporting to VigiBase employ the scale, reflecting its low‑cost nature-no special software or lab tests are required.
Future Directions
While the Naranjo Scale will likely stay a cornerstone, experts anticipate two major evolutions:
- Ethical updates. The International Council for Harmonisation (ICH) draft guidance (2024) proposes replacing the placebo‑challenge question with a “therapeutic drug monitoring” item, aligning the tool with modern standards.
- AI‑augmented scoring. Emerging models such as the FDA’s Sentinel Initiative can ingest real‑world data and output probabilistic ADR scores. These are unlikely to replace Naranjo outright but will serve as a complementary layer for complex biologics and gene therapies.
For now, mastering the classic ten‑question format remains the most practical way for clinicians to contribute high‑quality safety data.
Quick Checklist for Busy Clinicians
- Verify the temporal relationship (Q2) - it carries the highest weight.
- Document any alternative causes (Q5) with supporting evidence.
- If rechallenge or placebo isn’t feasible, note the ethical reason and choose “Do Not Know.”
- Cross‑check the final score with the category table before reporting.
- Consider a second tool for polypharmacy or pediatric cases.
What is the main advantage of using the Naranjo Scale?
It converts a subjective clinical judgment into a numeric score, making ADR assessments reproducible and easier to report to regulators.
Can the Naranjo Scale be used for multiple drugs at once?
No. The original design evaluates a single suspect drug. For polypharmacy, tools like the Liverpool ADR Probability Scale or PADRAT are recommended.
How should I handle a “Do Not Know” answer for the placebo challenge?
Select “Do Not Know” and clearly note in the case narrative that ethical constraints prevent a placebo rechallenge. This avoids inflating the score.
Is there an official digital version endorsed by regulatory agencies?
Regulators do not endorse a single software, but many EHR vendors (e.g., Epic) embed the questionnaire, and open‑source calculators such as the GitHub Naranjo app are widely accepted.
What score range defines a “definite” ADR?
A total score of 9 or higher indicates a definite adverse drug reaction.
9 Comments
Write a comment
More Articles
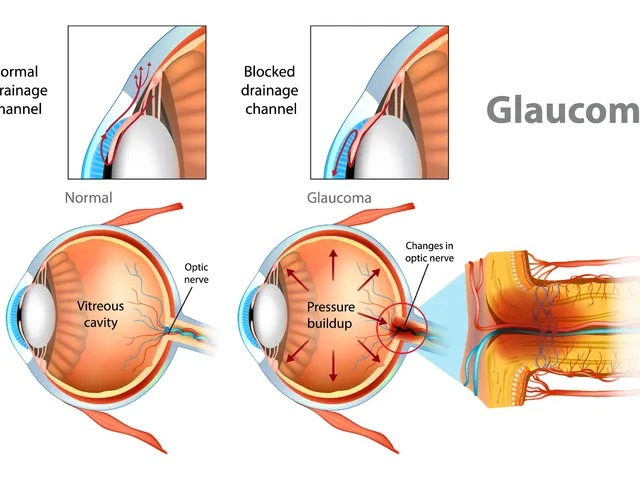
The effectiveness of dorzolamide in treating different types of glaucoma
Hey there, gentlemen! I just wanted to share some insights on a very interesting topic - the effectiveness of dorzolamide in treating different types of glaucoma. We'll dive into its mechanism of action, its beneficial effects on different types of this eye condition, and why it's becoming a go-to medication. Through this, I hope to provide everyone with a deeper understanding of this treatment option in a clear and simple language. Remember, knowledge is your best ally in health matters!

Molybdenum: The Essential Dietary Supplement for a Healthier You
In my recent blog post, I explored the importance of Molybdenum, a lesser-known but essential dietary supplement. I found that it plays a crucial role in many of our body's biological processes, including metabolic function, enzyme activity, and overall cellular health. Furthermore, a deficiency in Molybdenum can lead to serious health problems, underscoring its importance in our diet. However, it's also key to note that it should be taken in moderation as an excess can also cause health issues. So, in the pursuit of a healthier you, consider the balance of Molybdenum in your diet.

Anurag Ranjan
October 25, 2025 AT 20:45The Naranjo Scale is a quick way to turn a gut feeling into a reproducible score, making ADR reporting less guesswork.