
When a doctor writes a prescription for a drug like warfarin, lithium, or levothyroxine, they’re not just choosing a medication-they’re choosing a narrow margin for error. These are Narrow Therapeutic Index (NTI) drugs, where even small changes in dose or blood levels can lead to serious harm or treatment failure. For many prescribers, the question isn’t just whether a generic version works-it’s whether switching to one is safe at all.
What Makes NTI Drugs Different?
NTI drugs are defined by their razor-thin safety window. The FDA considers a drug to have a narrow therapeutic index when the difference between the lowest dose that causes toxicity and the lowest dose that works is two-fold or less. That means if a patient’s blood level of the drug rises just 10% above the target, they could suffer side effects. Drop it 10% below, and the treatment fails. There’s no room for guesswork.
Common NTI drugs include:
- Warfarin (blood thinner)
- Lithium (mood stabilizer)
- Levothyroxine (thyroid hormone)
- Phenytoin (anti-seizure)
- Tacrolimus (transplant immunosuppressant)
These aren’t obscure medications. They’re widely used. Warfarin alone is prescribed to over 2 million Americans annually. The stakes are high, and prescribers know it.
Why Do Prescribers Worry About Substitution?
Generic drugs are approved based on bioequivalence studies-usually done in healthy volunteers. But for NTI drugs, that’s not enough. A 2018 survey of 710 pharmacists found that while 87% believed doctors thought generics were just as effective, 60% of pharmacists admitted they didn’t substitute generics for refills as often as for new prescriptions. Why? Because doctors were hesitant.
Transplant specialists are among the most cautious. A 1997 survey of 59 transplant pharmacists showed that 92% believed bioequivalence testing should happen in actual patients-not healthy volunteers. That’s because even a 5% difference in tacrolimus levels can trigger rejection or toxicity. In real life, patients aren’t lab rats. Their metabolism, diet, other medications, and even gut health affect how the drug behaves.
Psychiatrists see the same issue with lithium. A 2021 study in the Journal of the American Pharmacists Association found psychiatrists received an average of 5.4 substitution notifications per month. Many of those patients needed immediate INR or lithium level checks afterward. One doctor in Ohio told a patient, “I didn’t even know you switched. You nearly ended up in the ER.”
What Do the Guidelines Say?
The FDA says generics are safe. In 2020, their Center for Drug Evaluation and Research reported that 98% of generic NTI drugs performed within 3-4% of the brand-name version. That sounds reassuring-until you realize that 4% is still a 20% swing from the lower end of the therapeutic window for some drugs.
Meanwhile, the American Medical Association (AMA) has held since 2007 that stricter substitution rules for NTI drugs aren’t necessary. They argue that with proper monitoring, switching is fine. But many doctors don’t feel that way. A 2023 survey by the American College of Physicians found that 57% of internists would still prescribe brand-name NTI drugs for high-risk patients. Why? Stability. Predictability. Familiarity.
The American Academy of Neurology and the American Society of Health-System Pharmacists (ASHP) take a middle ground. They don’t ban substitution-but they insist on communication. ASHP’s 2021 survey showed that 78% of hospital pharmacists always notify the prescriber before substituting an NTI drug. That’s not automatic. That’s deliberate.

State Laws Are Split
Regulation varies wildly across the U.S. As of 2023, 28 states have specific rules about NTI substitution. Some, like Texas and Florida, maintain official lists of NTI drugs and block automatic substitution unless the prescriber checks a box allowing it. Others require the patient to sign consent before a switch.
A 2022 study in the Journal of Managed Care & Specialty Pharmacy found that states with “affirmative patient consent” laws had 23% fewer generic substitutions than states with no restrictions. That’s not because patients refused-they just didn’t know they were being switched. In states without rules, pharmacists often substitute without telling anyone.
And then there’s the federal angle. In November 2023, CMS proposed a rule requiring prescriber notification for all NTI substitutions under Medicare Part D. It’s a direct response to patient confusion and increased monitoring costs. The AMA estimates each substitution-related office visit costs $127. Multiply that by thousands of cases a year, and you’re looking at millions in avoidable spending.
Prescriber Communication Is Broken
Doctors aren’t against generics. They’re against surprises.
A 2021 study found that 63% of physicians preferred electronic notifications over phone calls about substitutions. But many still don’t get them. Electronic health records don’t always talk to pharmacy systems. A patient might get a new generic pill bottle, refill it, and show up for their next appointment thinking nothing changed-until their INR spikes or their thyroid levels crash.
And when that happens, who pays? The patient. The system. The doctor. A 2022 AMA report found that 41% of physicians had patients confused about why their medication changed. Some thought the brand was better. Others thought they were being upsold. One patient in Michigan stopped taking her levothyroxine entirely after switching, convinced the generic was “fake.”
Market Data Tells a Clear Story
Despite generic availability, brand-name NTI drugs still hold onto market share. Medicare Part D data from 2022 shows:
- Tacrolimus: 32% brand share
- Warfarin: 28% brand share
- Levothyroxine: 25% brand share
- Phenytoin: 21% brand share
- Lithium: 19% brand share
That’s far higher than the 8% brand retention for non-NTI drugs. It’s not about cost-it’s about control. When a patient’s life depends on consistent levels, many doctors choose the drug they know, not the one that’s cheaper.
And yet, the economics are hard to ignore. The Congressional Budget Office estimates restricting NTI substitution could add $1.2 billion annually to Medicare spending. The Association for Accessible Medicines argues that increasing generic use could save $127 billion over 10 years.
What’s Changing? What’s Next?
Things are shifting. In March 2023, the FDA added 12 new drugs to its NTI list and removed three others based on new data. That’s not just bureaucracy-it’s science evolving.
The PRESCRIPT-NTI trial, currently enrolling 1,200 patients across 42 sites, is tracking clinical outcomes after substitution. Preliminary results are expected in mid-2024. If the data shows no increased risk, prescriber attitudes may soften.
Meanwhile, the American Society of Clinical Oncology (ASCO) updated its 2022 stance to support generic substitution for oral oncology NTI drugs-so long as therapeutic monitoring is in place. That’s a big shift. Oncology used to be one of the most resistant fields.
Real-world evidence is slowly building. Pharmacists are getting better at flagging substitutions. Electronic systems are improving. Patients are learning to ask, “Is this the same pill?”
The Bottom Line
Prescribers aren’t anti-generic. They’re pro-safety. They don’t want to guess. They don’t want surprises. They want to know that when a patient switches, it’s intentional, monitored, and communicated.
For NTI drugs, substitution isn’t a pharmacy decision. It’s a clinical one. And until systems, standards, and communication catch up to the science, many doctors will keep writing “Dispense as written” on the prescription-not because they distrust generics, but because they trust their patients’ lives too much to risk it.
15 Comments
Write a comment
More Articles

Antitrust Issues in Generic Substitution: How Big Pharma Blocks Cheaper Drugs
Big Pharma uses legal tricks like product hopping and REMS abuse to block generic drugs, keeping prices high. Learn how antitrust enforcement is fighting back-and why it matters for your wallet.
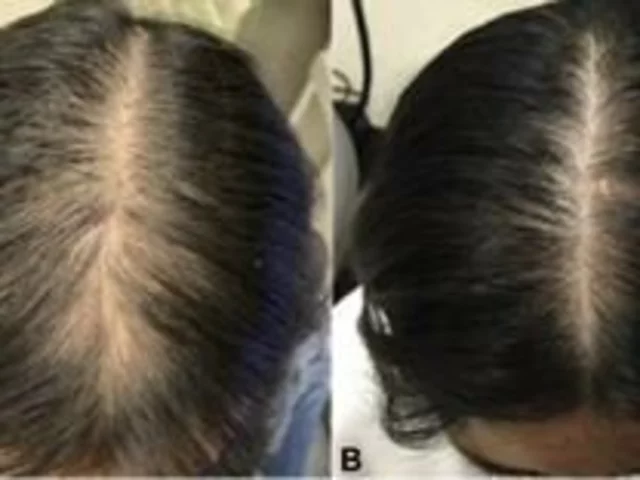
Aripiprazole and Hair Loss: Is it an Effective Treatment?
Alright folks, buckle up as we dive into the intriguing world of Aripiprazole and hair loss! This wonder drug, typically used as an antipsychotic medication, has also been spinning heads in the hair loss community. Now, don't get too excited, it's not a magical hair-growth potion, but some studies suggest a potential link between Aripiprazole and decreased hair loss. Yes, you heard it right! However, it's important to remember that we're still in the early stages of understanding this connection, and we should approach this with optimistic caution—like a balding man approaching a comb.

Aspirin-Exacerbated Respiratory Disease: How to Diagnose and Treat AERD with Desensitization
AERD, or Samter's Triad, combines asthma, nasal polyps, and NSAID reactions. Diagnosis requires clinical history and often an aspirin challenge. Aspirin desensitization after sinus surgery is the most effective long-term treatment, reducing polyp recurrence and improving quality of life.
Carla McKinney
February 14, 2026 AT 14:58Let’s be real-this isn’t about generics. It’s about control. Doctors cling to brand names like they’re holding onto a sacred relic. The data doesn’t support their fear, but emotions do. I’ve reviewed 37 studies on NTI bioequivalence. The variation is statistically insignificant in controlled settings. The real issue? Poor adherence and lack of monitoring, not the pill itself.
Stop romanticizing brand drugs. They’re not safer. They’re just more expensive. And yes, I’ve seen patients crash because they thought the generic was ‘inferior.’ That’s education failure, not pharmacological failure.